HHS partners to develop faster tests to identify bacterial infections
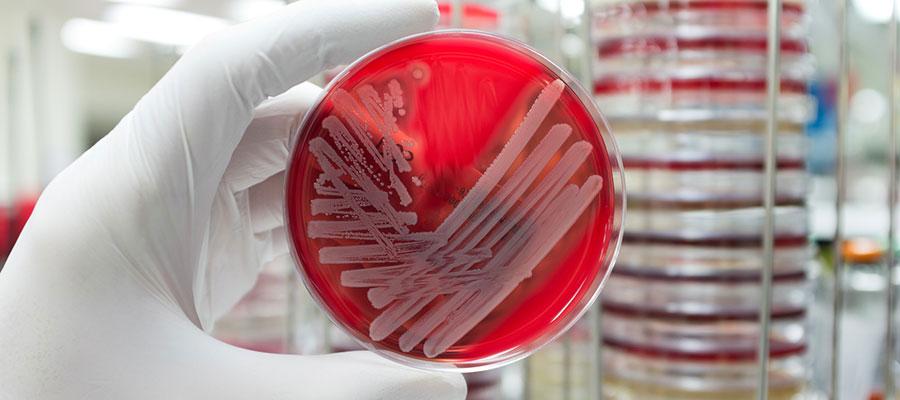
The Department of Health and Human Services’ Office of the Assistant Secretary for Preparedness and Response yesterday announced a $9.3 million contract to develop a test that could help hospital and commercial laboratories speed diagnosis of bacterial infections and determine the best antibiotics to treat them. ASPR’s Biomedical Advanced Research and Development Authority will sponsor advanced development of the testing technology under an 18-month contract with SeLux Diagnostic Inc. The agency said the technology aims to reduce the time needed to diagnose bacterial infections from days to hours and curb antibiotic-resistant infections.
Related News Articles
Headline
Antimicrobial-resistant infections remained above pre-pandemic levels in 2022, the Centers for Disease Control and Prevention reported July 16. CDC data show…
Headline
The Centers for Disease Control and Prevention July 14 announced four confirmed human cases of H5N1 bird flu among farm workers who were working at a Colorado…
Headline
The Administration for Strategic Preparedness and Response June 25 announced a flu pandemic preparedness and response strategy in response to the threat of…
Headline
The Centers for Disease Control and Prevention June 25 issued a Health Alert Network Health Advisory about an increased risk of dengue virus infections in the…
Headline
The Food and Drug Administration last week granted enforcement discretion for the use of conjunctival swabs by laboratories as part of human testing for H5N1…
Headline
The Centers for Disease Control and Prevention May 21 announced recommendations that flu surveillance systems continue operating at enhanced levels during the…

