CDC recommends updated COVID-19 boosters
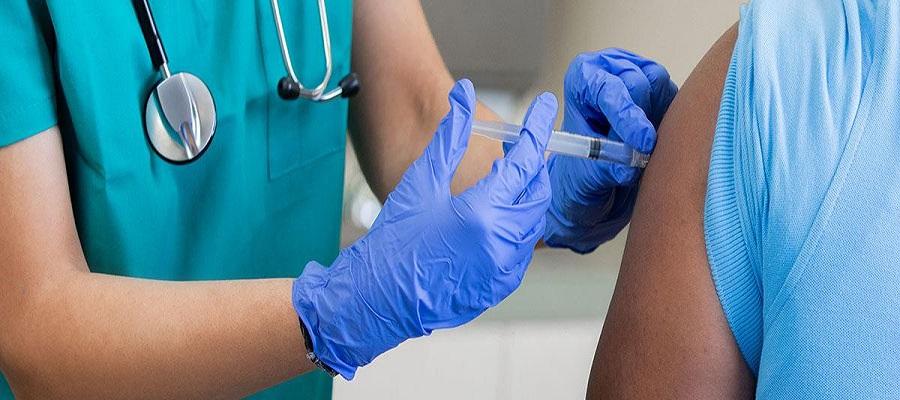
The Centers for Disease Control and Prevention last week recommended Pfizer’s updated COVID-19 vaccine booster for Americans aged 12 and older and Moderna’s updated COVID-19 vaccine booster for Americans aged 18 and older, as recommended by its vaccine advisory committee. Authorized by the Food and Drug Administration Aug. 31, the updated boosters are bivalent, meaning they help protect against the most recently circulating omicron variants as well as the original virus strain.
“This recommendation followed a comprehensive scientific evaluation and robust scientific discussion,” said CDC Director Rochelle Walensky, M.D. “If you are eligible, there is no bad time to get your COVID-19 booster and I strongly encourage you to receive it.”
Related News Articles
Headline
The Senate Finance Committee Feb. 4 voted 14-13 to advance Robert F. Kennedy Jr.’s nomination for secretary of the Department of Health and Human Services. A…
Headline
Respiratory illness activity remains high across the country, according to the latest data from the Centers for Disease Control and Prevention. Seasonal flu…
Headline
The Occupational Safety and Health Administration Jan. 13 announced that it terminated efforts to establish a final COVID-19 safety standard to protect workers…
Headline
The Department of Health and Human Services Dec. 10 amended the Public Readiness and Emergency Preparedness Act declaration for COVID-19, extending liability…
Headline
AHA's latest social media toolkit for encouraging vaccination against the flu and COVID-19 provides fall-themed social media posts and graphics. Download the…
Headline
The Centers for Disease Control and Prevention last week endorsed a recommendation for people aged 65 and older and for immunocompromised individuals to…

