States begin ordering more monkeypox vaccine
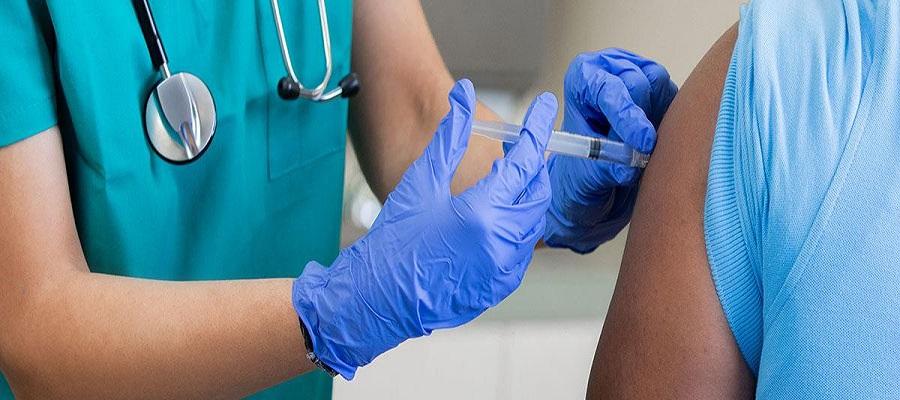
The Department of Health and Human Services Monday began accepting orders from states and other jurisdictions for a portion of 442,000 additional doses of the JYNNEOS monkeypox vaccine. To stretch available doses up to five fold, the Food and Drug Administration last week issued an emergency use authorization allowing health care providers to administer the vaccine intradermally to adults. HHS previously allocated almost 1.1 million doses of the vaccine to jurisdictions, and expects the manufacturer to deliver another 5 million doses through next year. HHS Secretary Xavier Becerra this month declared the U.S. monkeypox outbreak, totaling almost 12,700 cases since May, a public health emergency.
Related News Articles
Headline
As summer Pride parades and other large gatherings approach, Americans at high risk for mpox (monkeypox) exposure should ensure they are fully vaccinated with…
Headline
The Food and Drug Administration Friday authorized for emergency use a molecular test to detect mpox (formerly known as monkeypox) at the point of care in…
Headline
The Food and Drug Administration Friday authorized for emergency use a real-time polymerase chain reaction test to detect mpox (formerly known as…
Headline
In a study of men under age 50 who were eligible to receive the JYNNEOS vaccine to prevent monkeypox (mpox) during the U.S. outbreak this year, unvaccinated…
Headline
The World Health Organization today recommended a new name for monkeypox that is intended to mitigate a rise in related racist and stigmatizing language…
Headline
The Department of Health and Human Services today renewed the nation’s monkeypox public health emergency declaration for another 90 days. When the PHE was…

