Pfizer: COVID-19 vaccine still 100% effective in adolescents after four months
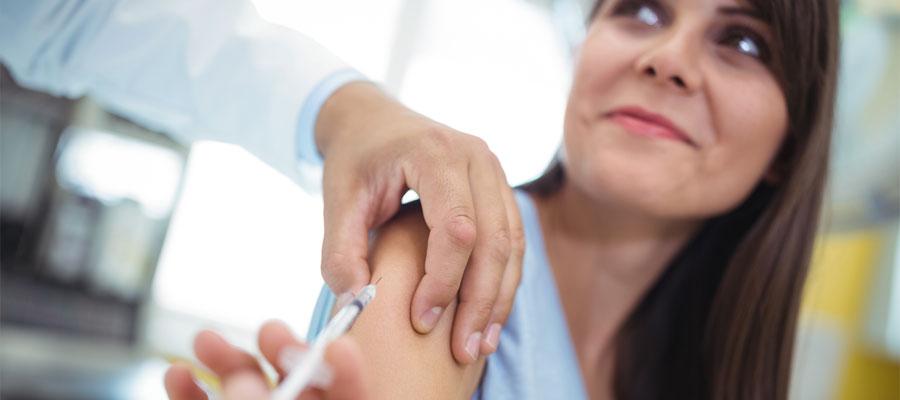
A two-dose series of the Pfizer-BioNTech COVID-19 vaccine was 100% effective against COVID-19 in 12-15 year olds more than four months after the second dose in the companies’ phase 3 clinical trial, Pfizer and BioNTech announced yesterday. The adverse event profile was generally consistent with other clinical safety data for the vaccine, with no serious safety concerns observed in individuals with at least six months of safety follow-up after the second dose, the companies said. They plan to submit the updated data to the Food and Drug Administration, which in May expanded the vaccine’s emergency use authorization to include 12-15 year olds.
Related News Articles
Headline
A study published April 8 by the Public Library of Science’s Journal of Global Public Health found that driving while infected with COVID-19 raises the risk of…
Headline
The Senate Finance Committee Feb. 4 voted 14-13 to advance Robert F. Kennedy Jr.’s nomination for secretary of the Department of Health and Human Services. A…
Headline
Respiratory illness activity remains high across the country, according to the latest data from the Centers for Disease Control and Prevention. Seasonal flu…
Headline
The Occupational Safety and Health Administration Jan. 13 announced that it terminated efforts to establish a final COVID-19 safety standard to protect workers…
Headline
The Department of Health and Human Services Dec. 10 amended the Public Readiness and Emergency Preparedness Act declaration for COVID-19, extending liability…
Headline
AHA's latest social media toolkit for encouraging vaccination against the flu and COVID-19 provides fall-themed social media posts and graphics. Download the…

